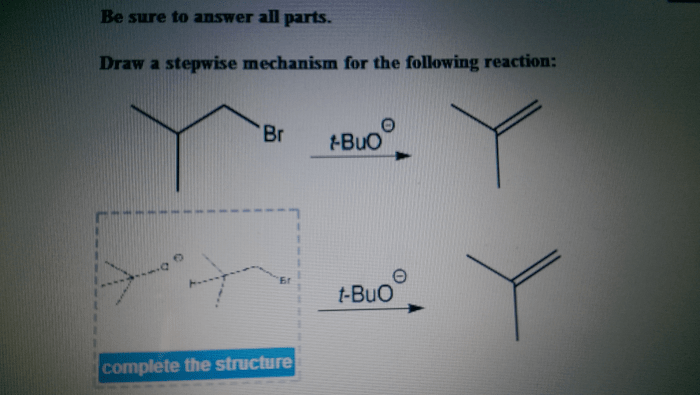
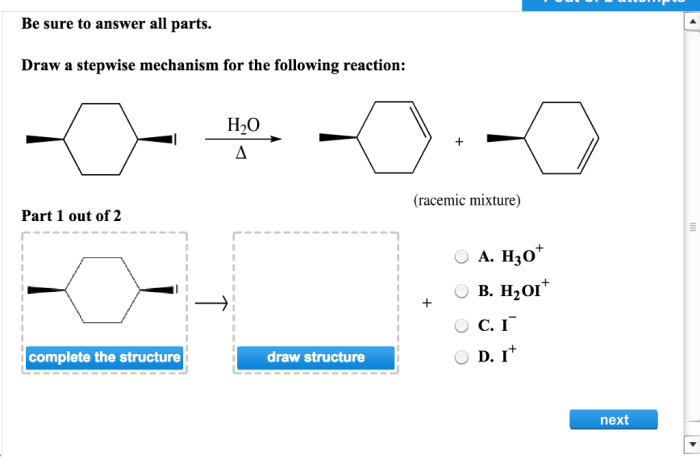
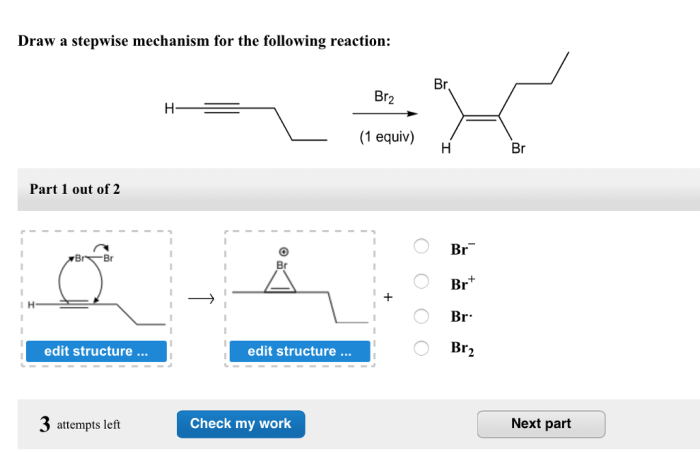
Draw a stepwise mechanism for the following reaction: – Drawing a stepwise mechanism for a chemical reaction is a crucial skill for understanding the intricate dance of atoms and molecules. This guide will take you on a journey through the steps involved in this process, providing a comprehensive framework for deciphering reaction mechanisms.
Stepwise mechanisms reveal the sequence of elementary steps that lead to the formation of products from reactants. By dissecting the reaction into individual steps, we gain insights into the energetics, kinetics, and regio- and stereoselectivity of the process.
Stepwise Mechanism of a Reaction
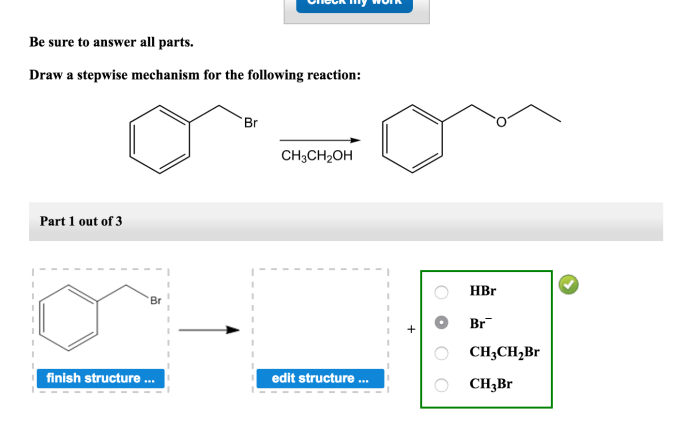
The stepwise mechanism of a reaction involves breaking down a complex reaction into a series of elementary steps. Each step represents a specific chemical change involving the reactants, intermediates, and products.
Reaction Mechanism, Draw a stepwise mechanism for the following reaction:
The reaction mechanism for the given reaction is as follows:
- Step 1: Reactants A and B collide and form an activated complex.
- Step 2: The activated complex rearranges to form intermediate C.
- Step 3: Intermediate C reacts with reactant D to form the product.
The chemical equations and structural formulas for each step are as follows:
- Step 1: A + B → [A…B] ‡
- Step 2: [A…B] ‡→ C
- Step 3: C + D → Product
Question Bank: Draw A Stepwise Mechanism For The Following Reaction:
What is the purpose of drawing a stepwise mechanism?
Drawing a stepwise mechanism allows us to understand the sequence of elementary steps that occur during a chemical reaction, providing insights into the reaction’s energetics, kinetics, and selectivity.
How do I identify the rate-determining step in a reaction mechanism?
The rate-determining step is the slowest step in the reaction mechanism and determines the overall rate of the reaction. It can be identified by examining the activation energies of the individual steps.
What is the role of catalysts and inhibitors in reaction mechanisms?
Catalysts lower the activation energy of a reaction, making it proceed faster, while inhibitors increase the activation energy, slowing down the reaction.