The purification of hydrogen gas by diffusion through a palladium membrane offers a promising solution for various industries. This technique leverages the unique properties of palladium to selectively permeate hydrogen molecules, enabling the removal of impurities and the production of high-purity hydrogen gas.
The palladium diffusion process involves the selective permeation of hydrogen molecules through a thin palladium membrane, while larger molecules and impurities are retained. This process is driven by a concentration gradient, with hydrogen molecules diffusing from a high-concentration region to a low-concentration region.
1. Introduction
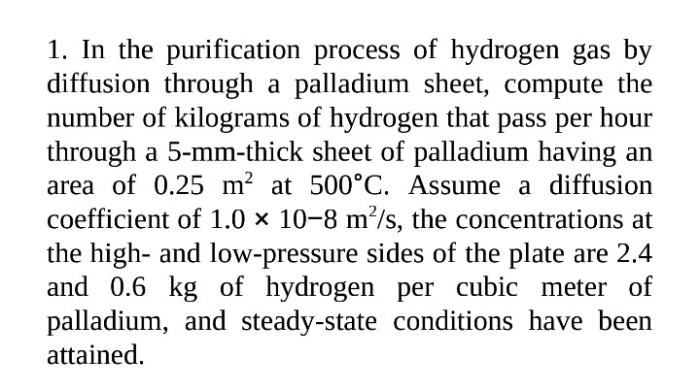
Hydrogen gas is an essential fuel source for various industries and applications. However, impurities in hydrogen gas can significantly impact its performance and safety. Hydrogen purification is crucial to remove these impurities and ensure the gas’s quality and reliability.
Palladium diffusion is a well-established technique for purifying hydrogen gas. This method utilizes the unique properties of palladium as a selective membrane, allowing hydrogen to permeate while blocking most other gases.
2. Palladium Diffusion Process: The Purification Of Hydrogen Gas By Diffusion Through A Palladium

Palladium is a metal with a face-centered cubic crystal structure. Its lattice structure creates small interstitial sites that allow hydrogen atoms to diffuse through while blocking larger molecules. This selective permeability makes palladium an ideal material for hydrogen purification.
The mechanism of hydrogen permeation through palladium involves the dissociation of hydrogen molecules (H2) into atomic hydrogen (H) at the surface of the membrane. These atomic hydrogen atoms then diffuse through the interstitial sites within the palladium lattice and recombine to form H2 molecules on the opposite side of the membrane.
Factors such as temperature and pressure affect the diffusion rate of hydrogen through palladium. Higher temperatures increase the kinetic energy of hydrogen atoms, leading to faster diffusion. Similarly, higher pressures create a concentration gradient that drives hydrogen permeation.
3. System Design and Operation
A typical palladium diffusion purification system consists of a palladium membrane module, a feed gas supply, and a permeate gas collection system. The feed gas containing hydrogen and impurities is passed through the palladium membrane module. Hydrogen selectively permeates through the membrane, leaving behind the impurities in the feed gas.
The operating parameters of a palladium diffusion system include the feed gas pressure, temperature, and flow rate. These parameters are optimized to achieve the desired purification efficiency and hydrogen recovery.
4. Performance Evaluation

The purification efficiency of a palladium diffusion system is typically evaluated based on the purity of the permeate gas. The purity can be measured using various analytical techniques, such as gas chromatography or mass spectrometry.
Factors influencing the purity of hydrogen gas include the feed gas composition, the operating parameters, and the membrane characteristics. Impurities with similar molecular sizes to hydrogen, such as helium, can compete with hydrogen for permeation, affecting the purification efficiency.
Palladium diffusion is often compared with other hydrogen purification methods, such as pressure swing adsorption (PSA) and cryogenic distillation. Each method has its advantages and disadvantages, and the choice depends on specific application requirements and cost considerations.
5. Applications and Case Studies
Palladium diffusion is widely used in various industries and applications, including:
- Electronics: Hydrogen purification for semiconductor manufacturing
- Fuel cells: Hydrogen purification for fuel cell vehicles
- Chemical industry: Hydrogen purification for ammonia synthesis
- Aerospace: Hydrogen purification for rocket propulsion
Case studies have demonstrated the successful implementation of palladium diffusion for hydrogen purification in these applications, leading to improved product quality, increased efficiency, and reduced environmental impact.
6. Future Trends and Research
Advancements in palladium membrane technology are focused on improving the membrane’s selectivity, durability, and cost-effectiveness. Research is also ongoing to explore emerging applications of hydrogen gas purification, such as hydrogen storage and transportation.
Areas for further research and development include the development of novel palladium alloys with enhanced hydrogen permeability, the optimization of membrane design and fabrication techniques, and the integration of palladium diffusion with other purification technologies.
Question Bank
What are the key factors affecting the diffusion rate of hydrogen through palladium?
Temperature, pressure, and the thickness of the palladium membrane are the primary factors influencing the diffusion rate.
How does the purity of the hydrogen gas affect its performance in fuel cells?
High-purity hydrogen gas is crucial for optimal fuel cell performance, as impurities can poison the catalyst and reduce efficiency.
What are the advantages of using palladium diffusion over other hydrogen purification methods?
Palladium diffusion offers high selectivity, efficiency, and a compact design, making it suitable for various applications.